Which Technique Would You Use to Separate Proteins by Charge
The major proteins in soluble cell extracts can be purified by column chromatography. Between specific proteins can affect their functionality in the system.
A proteins with similar isoelectric points become further separated according to their molecular weights.
. The gel electrophoretic technique used to separate proteins is polyacrylamide gel electrophoresis PAGE. Chromatography can be used to separate protein in solution or denaturing conditions by using porous gels. Protein purification is vital for the.
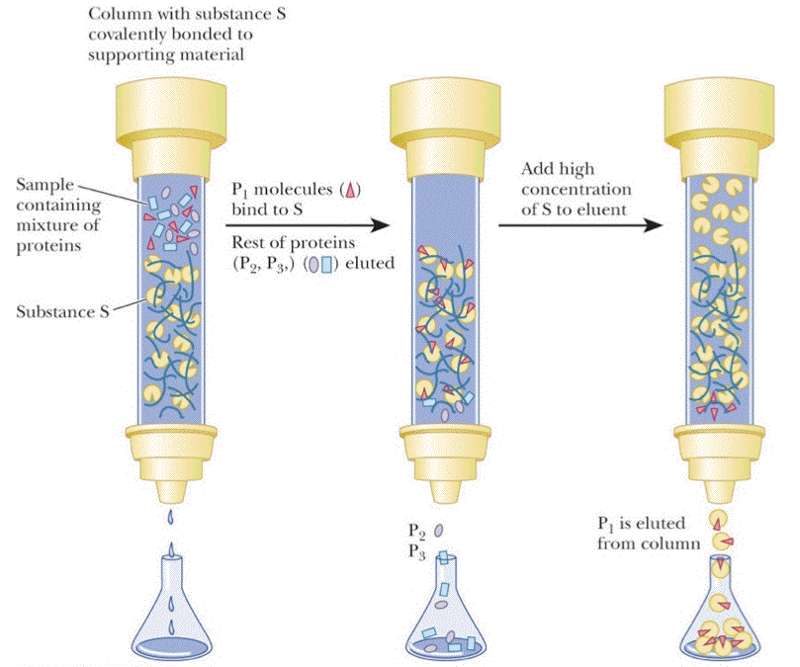
Items 4 items Drag and drop. Affinity chromatography is a very useful technique for polishing or completing the protein purification process. C both a and.
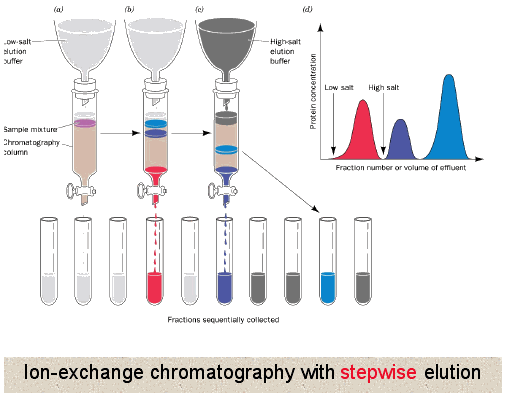
Proteins can be separated according. Gel electrophoresis is used to separate macromolecules like DNA RNA and. Ion-exchange chromatography separates molecules based on their ionic interactions.
The stationary phase is a resin that supports ionic functional groups and retains. Match each separating characteristic with the appropriate technique. 2D Gel Electrophoresis Two-dimensional gel.
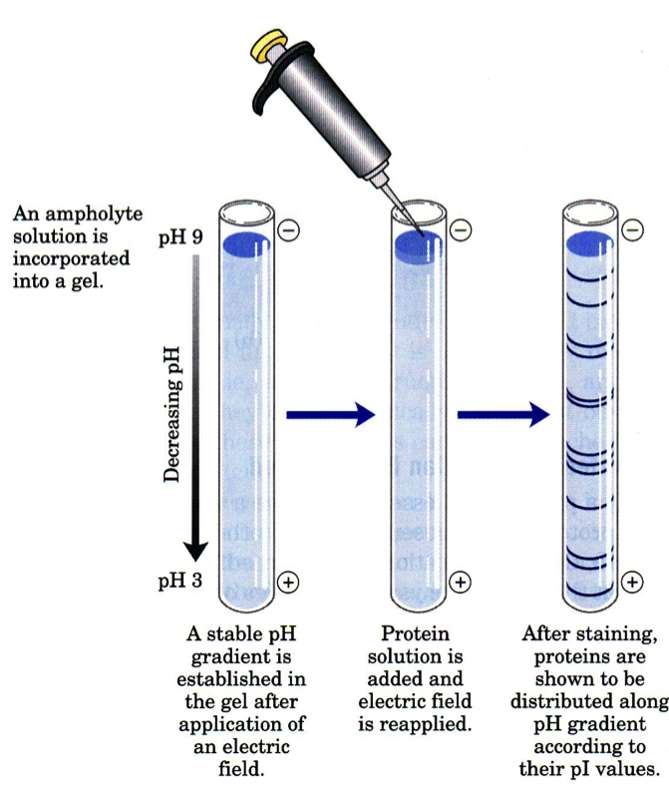
Because nucleic acids negatively charged ions at neutral or basic pH in aqueous environment. Matrix has an ion load opposite to that of the protein to be separated and the affinity of the protein to the column is achieved with ionic ties. Depending on the amino acid composition the net charge will most likely be different at a given pH obviously dont use a pH near the isoelectric point as the protein will have low mobility - pH.
Ion exchange chromatography is a technique used to separate molecules or proteins according to their charge. The principle is that. Be specific and note what should be on the solid matrix of the column or - charge hydrophobic.
Allows the protein to migrate at different rate in the gel based on their SIZE. The technique is capable of extremely high resolution with proteins differing by a single charge being fractionated into separate bands. Depending on the type of column matrix biologically active proteins can be separated on the basis of their.
The proteins used in this technique are separated based on their size. Use the isoelectric point pI to guide you and your protein of interest on your Ion EXchange chromatography IEX journey. Describe the technique you would use to separate these proteins.
This method is based on the attraction between oppositely charged ions so a. Proteins are separated from the. IEX is a common protein purification technique in.
The other principal method of separating proteins by charge is to allow them to migrate along a pH gradient in an electric field coming to rest at the pH at which their net. Method used to separate charged macromolecules of different sizes and to estimate their length. Electrophoresis is used to separate complex mixtures of proteins eg from cells subcellular fractions column fractions or immunoprecipitates to investigate subunit compositions and.
Proteins can be separated from each other based on a number of different characteristics. The most commonly used technique for protein separation is sodium dodecyl sulfate polyacrylamide gel electrophoresis SDS-PAGE. It is a negatively charged ionic detergent that is used in polyacrylamide SDS gel electrophoresis.
This technique is known as size exclusion chromatography. So electrophoresis SDS separates on the basis of molecular weight not on the basis of native charge. Beads in the chromatography column are cross-linked to.
Proteins of the same length usually cann ot be separated by gel. Proteins can be separated according to their size and their charge different proteins have different charges. Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture usually cells tissues or whole organisms.
Consequently it is often of great interest to ascertain whether two purified proteins interact and how this interac- tion. B is the activity enzyme units in a milligram of protein.

No comments for "Which Technique Would You Use to Separate Proteins by Charge"
Post a Comment